Morphological Characteristics of the Eurasian Lynx (Lynx lynx)
BODY: feline, 80–130 centimetres long
LEGS: long, with forelegs significantly shorter than hind legs
TAIL: short, 10–30 centimetres
BODY WEIGHT: adult animals (data from Slovenia): females 17.9 ±3.0 kg; males 21.5 ± 3.3 kg
SKULL: short, feline
DENTAL FORMULA: 28 teeth in total: I 3/3, C 1/1, P 2/2, M 1/1
FUR: thick and woolly, base colour is light brown, somewhat darker over the back than over the flanks, and white with a yellow tint on the inner side of legs and over the abdomen; the lynx has a characteristic spotted pigmentation, with an individually specific number and distribution of spots.
SENSES: vision, especially at night, is extremely good; the sense of smell is good, but not as good as in case of dogs; hearing is very good and over a very wide frequency range.
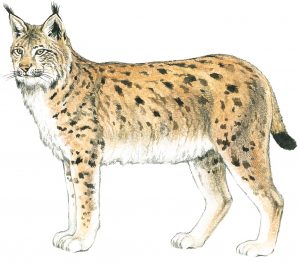
Figure: Igor Pičulin

Figure: Igor Pičulin
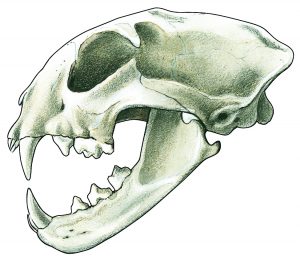
Figure: Igor Pičulin
Sources:
Kos I., Potočnik H., Skrbinšek T., Skrbinšek M.A., Jonozovič M., Krofel M. 2004. Ris v Sloveniji. 1. izd. Ljubljana, Biotehniška fakulteta, Oddelek za biologijo: 239 str. (Lynx in Slovenia. Ljubljana, Biotechnical Faculty, Department of Biology. 239 pg.)

Foto: Miha Krofel
Habitat
In Europe, the Eurasian lynx (Lynx lynx) inhabits predominantly deciduous, coniferous and mixed forests. The vegetation cover plays an important role in lynx home range selection for various reasons: it is a crucial habitat component of the prey populations; it represents a refuge against anthropogenic disturbance; and it provides cover for hunting.
Lynx habitat selection is primarily driven by avoidance of human activity during day and prey availability during night. Night ranges exceed day ranges by more than 10%. At night, lynx select open habitats, such as meadows, which are associated with high ungulate abundance at that time. By contrast, during the day, lynx select habitats offering dense understorey cover and rugged terrain away from human infrastructure.
With a higher density of people, the likelihood of human disturbance increases for lynx. An important limiting factor is transportation infrastructure that fragments suitable habitat. Fragmentation is also caused by larger areas of inadequate space, such as urban areas and natural barriers, like large rivers and high mountain ridges.
Sources:
Kos I., Potočnik H., Skrbinšek T., Skrbinšek M.A., Jonozovič M., Krofel M. 2004. Ris v Sloveniji. 1. izd. Ljubljana, Biotehniška fakulteta, Oddelek za biologijo: 239 str. (Lynx in Slovenia. Ljubljana, Biotechnical Faculty, Department of Biology. 239 pg.)
Filla M., Premier J., Magg N., Dupke K., Khorozyan I., Waltert M., Bufka L., Heurich M. 2017. Habitat selection by Eurasian lynx (Lynx lynx) is primarily driven by avoidance of human activity during day and prey availability during night. Ecology and evolution, DOI: 10.1002/ece3.3204.
Strategija ohranjanja in trajnostnega upravljanja navadnega risa (Lynx lynx) v Sloveniji 2016–2026: 41 str.
http://www.mop.gov.si/fileadmin/mop.gov.si/pageuploads/podrocja/velike_zveri/strategija_ris_2016_2026.pdf (December 2017)

Foto: Miha Krofel
Spatial Organization and Territoriality
Lynx, with the exception of females with kittens of less than one year, live a solitary life within a specific home range. Females compete for resources required to raise kittens, and are relatively evenly distributed over the space. Males don’t participate in parental care, but compete for females during the breeding season. That is why their home ranges overlap with the home ranges of females, while they try to keep other males away.
Sizes of yearly home ranges in Western and Central Europe (data from Switzerland; data from Poland was similar): males: 264 ± 23 km2, females: 168 ± 64 km2.
Lynx are a territorial species, where each male defends his territory against other males and each female defends her territory against other females. Their social organization is similar to other solitary felids, displaying intrasexual territoriality with limited overlap on the periphery and overlapping home ranges between the sexes. Lynx are polygamous and monoestrous with peak of mating in late winter. During the breeding period, extra-territorial excursions of males have been recorded, sometimes with successful mating within the neighbour’s territory. Aggressive intraspecific interactions are rare and usually occur between males during breeding season. The stability of land tenure system is believed to depend mainly on indirect communication, especially scent marking, which also plays a role in mate selection.
Crucial to spatial distribution is chemical communication via urine markings and cheek rubbing. Marking sites can serve as ‘chemical bulletin boards’, where lynx advertise their presence and gain information on ownership relationships in a given area. In Dinaric mountains, it was observed that lynx preferentially use forest roads for scent marking. Marking activity of resident animals peaks during the mating season and is lowest during the period when females give birth and lactate. Both sexes scent-mark, but male lynx visit marking sites much more often than females and mark relatively more often when visiting a site.
Sources:
Kos I., Potočnik H., Skrbinšek T., Skrbinšek M.A., Jonozovič M., Krofel M. 2004. Ris v Sloveniji. 1. izd. Ljubljana, Biotehniška fakulteta, Oddelek za biologijo: 239 str. (Lynx in Slovenia. Ljubljana, Biotechnical Faculty, Department of Biology. 239 pg.)
Vogt K., Zimmermann F., Kölliker M., Breitenmosera U. 2014. Scent-marking behaviour and social dynamics in a wild population of Eurasian lynx Lynx lynx. Behavioural Processes,106: 98–106.
Allen M.L., Hočevar L., Groot M., Krofel M. 2017. Where to leave amessage? The selection and adaptive significance of scent-marking sites for Eurasian lynx. Behav Ecol Sociobiol. DOI: 10.1007/s00265-017-2366-5.
Krofel, M., Hočevar, L., Allen, M.L. 2017. Does human infrastructure shape scent marking in a solitary felid? Mammalian Biology, 87: 36-39. DOI: 10.1016/j.mambio.2017.05.003

Foto: Miha Krofel
Feeding Habits
MAIN PREY
The main prey of lynx are roe deer, edible dormouse, red deer, chamois, and, in other parts of the range, caribou, fallow deer, and mouflon. Small prey might be especially important for young lynx after they separate from their mothers. The selection of prey greatly depends on its abundance. In Slovenia, lynx’s prey is primarily roe deer, edible dormouse and red deer. It hunts all categories of small ungulates, but selects young and/or weak red deer, primarily hinds and calves.
HUNTING TECHNIQUE
Since felids are mostly solitary hunters, each bite must be made with precision, and must be positioned to kill the prey as soon as possible to avoid risks to the predator during the struggle. An important factor for hunting success is surprise. It stalks its prey or attacks it from an ambush. To be successful, it must get within a few metres of its prey. The lynx does not chase the prey, and usually gives up if the attack does not succeed in 20–50 metres.
The lynx kills its prey with a bite to the throat, crushing the wind pipe, or with a bite into the nape of the neck, severing the spinal cord. It can kill small prey with a bite to the head. Bites from below into the region of the common carotid artery and truncus vagosympathicus (pressure on the carotid sinus with numerous baroreceptors can result in bradycardia or total cardiac arrest and immediate death) have also been registered.
CONSUMPTION OF PREY
In case of larger prey, lynx do not consume its whole kill at once. It usually eats large muscles (thighs, shoulders) first, and then continues on toward the smaller muscles. The prey’s digestive tract is not consumed. The time a lynx spends feeding on a single prey increases logarithmically with the weight of the prey. It also depends on the number, age, and size of lynx. In Slovenia and Croatia lynx are feeding on roe deer on average for 4.4 days, unless disturbed by bears or people. In Switzerland, lynx have been found to feed on an adult chamois for an average of 4.6 days, on a buck roe deer for 4.3 days and on a doe for 4 days. Researchers from Poland have found that lynx will feed on roe deer for an average of about 3 days, and on red deer for about 4 days. A lynx frequently leaves its prey (especially males) and returns after a few days. It often hides and camouflages the prey. When lynx are disturbed at kill site by people, they may abandon the kill before it is completely consumed. In contrast to common belief, lynx does not remove the head of its prey. This is done by the red fox (sometimes also when scavenging on lynx prey remains).
PREY UTILISATION AND REQUIREMENTS
In most cases, a lynx eats all edible tissue of the prey (80% of cases, consumption higher than 75%). In 19% of cases, the utilisation is partial (consumption 25–75%), and the lynx abandoned its prey without any known reason in 1% of cases. A lynx needs 2 ± 0.9 kg of food per day, if the time needed to find the next prey is taken into account. If we add searching and consumption times, a lynx male captures a prey every 5.9 ± 1.5 days, and a female every 5.2 ± 1.4 days. Thus, we can deduce that a lynx needs approximately 60–70 large prey animals (roe deer, red deer and similar) annually and an unknown quantity of smaller prey.
Sources:
Kos I., Potočnik H., Skrbinšek T., Skrbinšek M.A., Jonozovič M., Krofel M. 2004. Ris v Sloveniji. 1. izd. Ljubljana, Biotehniška fakulteta, Oddelek za biologijo: 239 str. (Lynx in Slovenia. Ljubljana, Biotechnical Faculty, Department of Biology. 239 pg.)
Krofel M., Skrbinšek T., Kljun F., Potočnik H., Kos I., 2009. The killing technique of Eurasian lynx. Belg. J. Zool, 139(1): 79-80.
Krofel, M., Kos, I., Jerina, K. 2012. The noble cats and the big bad scavengers: effects of dominant scavengers on solitary predators. Behavioral Ecology and Sociobiology, 66 (9): 1297-1304. DOI: 10.1007/s00265-012-1384-6
Krofel, M. 2012. Medvrstne interakcije povezane s plenjenjem pri evrazijskem risu (Lynx lynx) na območju severnih dinaridov. Doktorska disertacija. Univerza v Ljubljani, Biotehniška fakulteta, Ljubljana. 142 str.

Foto: Miha Krofel
Population Characteristics
The sex structure of stable lynx populations shows a balanced ratio between males and females. A larger proportion of males (64% in Finland) were found in areas of population expansion or population increase. This is caused by the larger dispersion rate of males.
It is assumed that cull data seriously underestimate the proportion of kittens in a population, as they are more exposed to other causes of mortality, such as diseases, predators, and adverse environmental factors. Culling usually becomes a cause of mortality only in the second half of their first year of life.
Based on lynx tracking data from Poland, researchers estimated that during the winter the population consists of 24–34% of adult males, 19–23% adult reproductive females, 11–26% subadult animals and non-reproductive females, and 20–45% kittens.
Lynx males in Europe reach sexual maturity by 33 months of age. Histological analyses of the testicles of the Norwegian lynx population show that approximately 50% of males reached sexual maturity by 21 months. The females in captivity also reach sexual maturity at 21 months. A similar result was obtained in Norway through analysis of ovulation or the presence of corpora lutea, where after approximately 21 months most females had reached sexual maturity. However, approximately half of the females had reached sexual maturity by the age of 7.5–11.5 months. In Scandinavia, for example, none of the 17 one-year old females included in the study delivered a litter.
It is not likely that young males would participate in mating, as they are chased away from females by older males. This probably means that they can mate only a year later, when they are almost 3 years old.
The size of litters in the Eurasian lynx is from one to four kittens. In Norway, they found 2.5 embryos per gravid female on average (SD ± 0.52). Kaczensky reported 2.1 (± 0.9) kittens per litter, in an interval from 1 to 4.
The reproductive success of a lynx population depends primarily on the feeding conditions in the environment, usually the density of roe deer. When they reach sexual maturity, Eurasian lynx females usually mate until the high age of 12–13 years. In a radiotelemetric study in Scandinavia they found that 55% of two-year old females and 72% of older females were with kittens.
Most European studies of lynx reproduction found the number of kittens per female at the end of their first year of life to be between 1.2 and 1.6. Deviating from this are data from Switzerland, where they found only 0.69 kittens per female in the studied animals.
The mortality of adult lynx is between 19% and 29%. The mortality of subadult and adult lynx in Poland during the years 1978 and 1994 was estimated to be 37%. Both in Poland and in Switzerland, 63% to 75% of known mortality was attributed to illegal hunting. An additional factor of human-caused mortality, typical for a man-dominated landscape, is vehicle-related collisions.
The survival of adult individuals has the most important impact on the population dynamic of the lynx. The second most important parameter is the survival of kittens up until their first year of age.
Estimates of lynx population densities in different parts of Europe range from 1 to 6 animals per 100 km2. In the Swiss Alps the density over a 500-km2 area was estimated at 1.2 adult lynx per 100 km2, with a higher density on the front of population expansion. The density in the Swiss Jura Mountains was 0.94 animals per 100 km2, and ranged between 1.4 and 1.9 animals per 100 km2, if the juvenile animals in autumn were also considered. The density of adult animals in autumn in the area of the Białowieża primeval forest during the 1991–1996 period was estimated to be between 2.4 and 3.2 animals per 100 km2. The density of kittens was estimated between 0.6 and 2.3 animals per 100 km2, with the resulting total density of 4 to 5 animals per 100 km2.
Sources:
Kos I., Potočnik H., Skrbinšek T., Skrbinšek M.A., Jonozovič M., Krofel M. 2004. Ris v Sloveniji. 1. izd. Ljubljana, Biotehniška fakulteta, Oddelek za biologijo: 239 str. (Lynx in Slovenia. Ljubljana, Biotechnical Faculty, Department of Biology. 239 pg.)
KACZENSKY, P. 1991. Untersuchungen zur Raumnutzung weiblicher Luchse (Lynx Lynx) sowie zur Abwanderung und Mortalitat ihrer Jungen im Schweizer Jura – Spatial use of female lynx, and dispersal and mortality of their off spring in the Swiss Jura. Universitat Munchen: 1 – 80.
